Forming Films and Top Webs
Flexible forming films can be a sustainable and cost effective alternative to rigid trays in many applications.
Available in thicknesses from 3 to 24 mil, in a range of tough materials.
Whether you need a tough nylon package, or a traditional ionomer one, our material engineers have choices for you.
All of our forming films are available In ‘standard’ format: With a traditional sealing surface designed to seal to a peelable top web, and In ‘direct seal’ format, with a peelable sealing surface designed to seal to non-peelable top webs, like uncoated Tyvek™. An uncoated top web can be a significant cost savings.
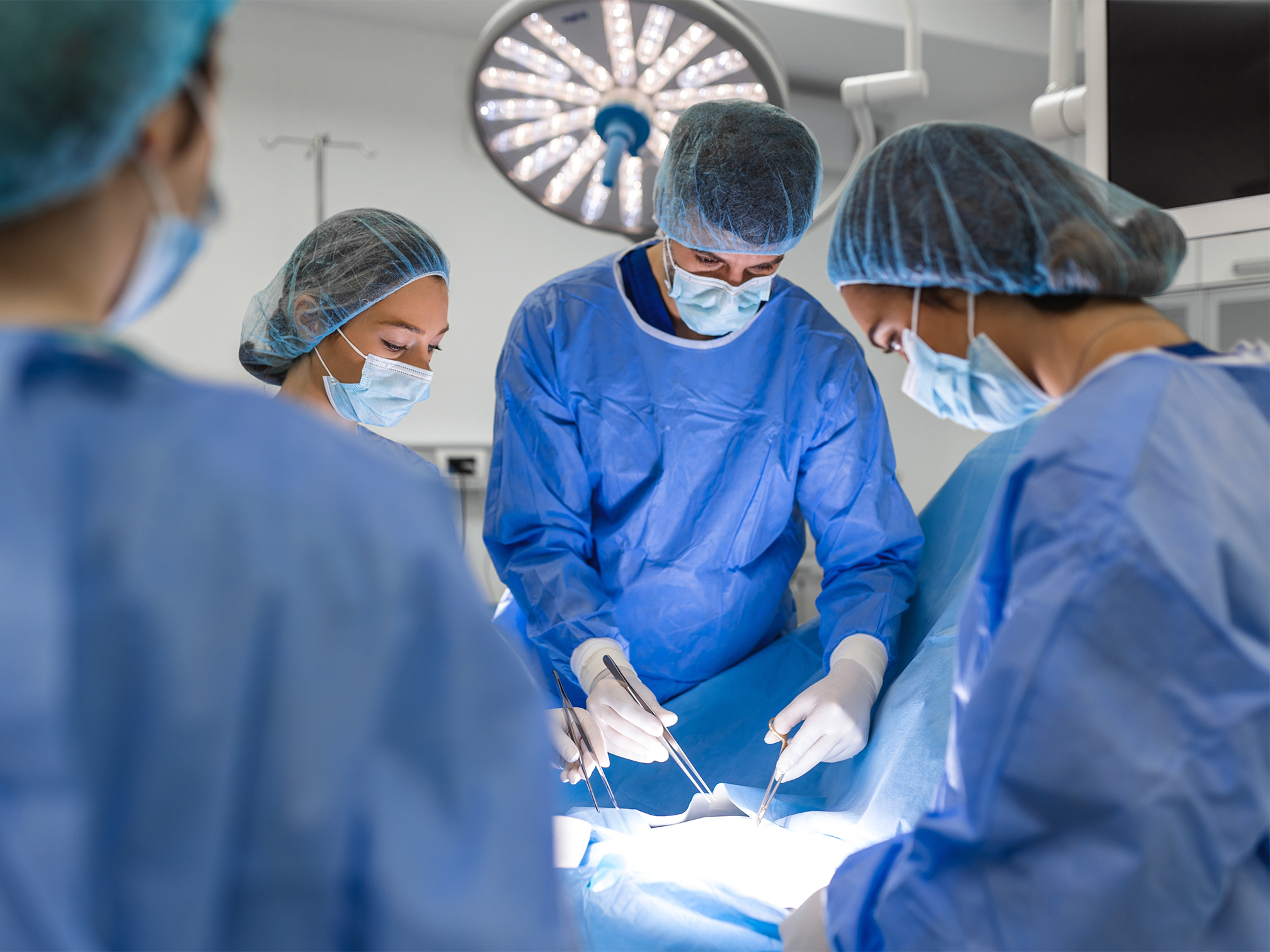
Our forming films are used in other applications beyond packaging, like surgical light handle covers.
We offer peelable and non-peelable top webs that match well to our forming films for secure, consistent seals.
• All medical grades of uncoated Tyvek™
• Paper
• Coex films
• Laminations in a range of materials
• Printed or unprinted
• We can design a custom top web for your application.
Laminations & Custom Packaging in Roll Form
While we offer many standard products in roll form for medical packaging – including peelable, surface printable and barrier laminations – most of our laminations are custom designed for each customer.
Let us create a lamination with the optimal set of properties for your application:
• Peelable or non-peelable
• Barrier (foil or foil alternative)
• Full color printed, or print ready
• Transparent or opaque
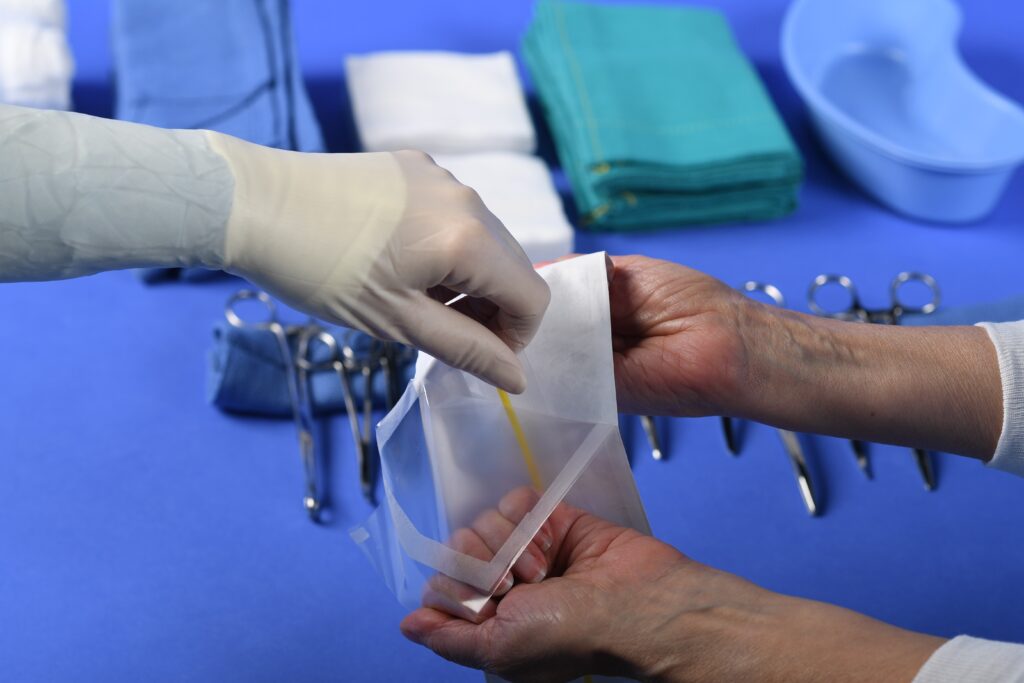
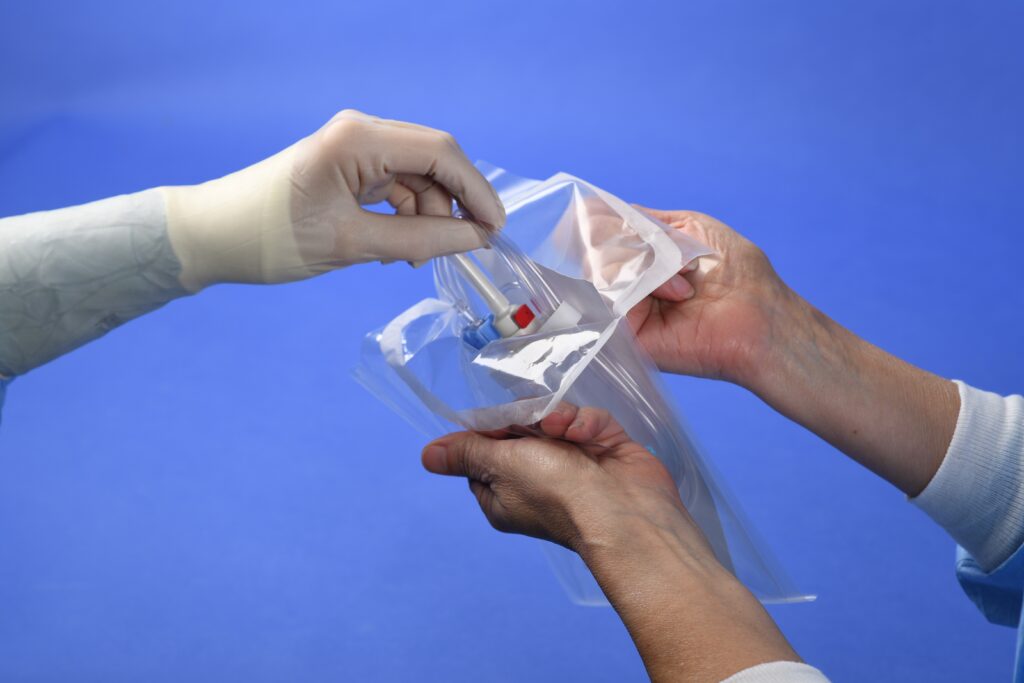
Premade Packages: Pouches and Bags
Whether you need a peelable or tear open pouch, or use gas or radiation sterilization, we have a pouch for your application.
We offer commonly used designs, materials and pouch sizes, but can design a custom package for your device.
Breathable Pouches
For a breathable pouch, we offer all Tyvek grades, plus paper options sealed to a clear polyester or nylon lamination.
Non-Breathable Pouches
If your device will be sterilized with radiation or e-beam, a Film-to-Film or Foil pouch can be a great option – available in peel open or tear open designs.
Header Bags
Header bags are a great choice for larger kits and contents that have an inner sterile barrier like a wrapper or back table cover. Mostly commonly used in a breathable format, our header bags are also designed in a non-breathable version for radiation sterilization.
Let our engineering team design a custom package for your application and sterilization method, recommending multiple options in materials and design.
Whether you need a tough end seal for heavy contents, or a peelable seal for lighter contents, you have choices.
Our film-to-film peel open seal design eliminates the risk of fiber tear when opening the bag.
Available in a range of sizes for small individual supplies to very large surgical kits.
Specialty Bags
In addition to header bags, we offer several unique bag designs.
• Header bag with an outer pocket, ideal for adding IFUs
• Linear tear bag, that easily tears open along the top edge
• Kwik tear bags, that easily snap open along the top edge
• Center strip bag, ideal for labware multipacks
All available in breathable and non-breathable designs and a range of materials.